SURGISPON® in Endoscopic Sinus Surgery: An Alternative to Traditional Nasal Packing
Endoscopic Sinus Surgery (ESS) has become the gold standard for treating chronic rhinosinusitis and nasal polyposis. With its minimally invasive approach and high success rates, ESS offers significant relief to patients. However, postoperative bleeding remains one of the most common and troublesome complications.
To address this, intranasal absorbable sponges for sinus surgery have become valuable tools for safe and efficient bleeding control.To prevent issues such as adhesion formation, middle turbinate lateralization, and restenosis, nasal packing is frequently used after ESS [1]. Traditionally, non-absorbable nasal packs have been employed for this purpose. While effective, they come with notable drawbacks:
- Painful removal that may cause mucosal trauma and rebleeding
- Patient discomfort due to nasal obstruction
- Increased risk of infections, foreign body reactions, or granuloma formation [2].
To overcome these limitations, absorbable nasal packing materials have emerged as a preferred alternative. The intranasal absorbable sponge for sinus surgery SURGISPON® offers a reliable combination of clinical effectiveness, biocompatibility, and cost-efficiency.
Why Choose Absorbable Gelatin Sponges for Nasal Packing?
Modern nasal surgeries emphasize patient comfort, safety, and faster recovery. Absorbable packing materials support these goals by eliminating the need for pack removal and minimizing trauma.
Commonly Used Absorbable Materials:
- Porcine gelatin matrix (e.g., Surgiflo®)
- Topical antifibrinolytics (e.g., Amicar®)
- Hyaluronic acid-based dressings
- Oxidized regenerated cellulose (e.g., Surgicel®)
- Polyethylene glycol-based sponges (e.g., Nasopore®)
These products have shown good results in reducing bleeding and adhesion formation. However, their major limitation is cost — many of these options are expensive and may not be accessible for all hospitals or healthcare settings [3].
This is where SURGISPON® offers a significant advantage — matching clinical performance at a more affordable cost.
Role of Intranasal Absorbable Sponge for Sinus Surgery
SURGISPON® is an absorbable haemostatic sponge designed for safe, effective nasal packing in ENT surgeries. It is a sterile, malleable, water-insoluble haemostatic sponge manufactured from highly purified pharmaceutical-grade gelatin. It is intended for haemostatic use by applying to bleeding surface and can be used either dry or saturated with sterile saline. SURGISPON® may also be used in conjunction with thrombin, although this is optional.SURGISPON®ENTis available in three sizes-NASAL STRIP (80x15x07 mm), NASAL TAMPON (80×07 mm) and Nasal Strip Compressed (80x15x02 mm).
Fig. 1: SURGISPON®Nasal Strip (80x15x07mm)
Fig.2: SURGISPON® Nasal Tampon (80×07 mm)

Fig. 3: SURGISPON® Nasal Strip Compressed (80x15x02 mm)
Key Features:
Mechanism of Action: How SURGISPON® Works
The haemostatic action of SURGISPON® is based on its porous structure, which activates platelets (thrombocytes) as soon as they come into contact with the sponge. This initiates:
- Platelet activation and aggregation
- Release of coagulation factors
- Acceleration of fibrin clot formation
SURGISPON® thus enhances the body’s natural coagulation cascade, leading to efficient local haemostasis without the need for external agents.
- In mucosal applications, SURGISPON® liquefies within 2–5 days
- Complete biodegradation occurs within 4 weeks when used in appropriate quantities
How to Use SURGISPON® in Nasal surgery
Soak the SURGISPON®Nasal Strip /Tampon in antibiotic, or sterile saline solution, can be used dry
- If used dry, the sponge is cut into the desired size and is slightly compressed. The sponges must be applied to a bleeding area under light pressure for one or two minutes until the bleeding stops
- If used wet, withdraw Nasal Strip /Tampon and squeeze thoroughly between gloved fingers to expel air bubbles. Replace in saline, and keep at bleeding site until needed
- When bleeding is controlled, Nasal Strip /Tampon can be left in situ
- When needed support is achieved, Nasal Strip /Tampon may be removed.
Clinical Evidence: Gelfoam® vs. No Packing in Sinus Surgery
A prospective, controlled study compared Gelfoam® absorbable gelatin sponge with no nasal packing after bilateral FESS in 21 patients with chronic rhinosinusitis.
Study Findings:
- Each patient had one nasal side packed with Gelfoam®, the other left unpacked.
- Postoperative bleeding occurred in 3 patients on the unpacked side, while no bleeding occurred on the Gelfoam® side.
- Healing parameters (crusting, granulation, synechiae) showed no significant difference between the two sides.
- Gelfoam® offered safe, effective haemostasis without requiring removal.
The study supports the clinical effectiveness of intranasal absorbable sponges for sinus surgery like SURGISPON® in providing safe haemostasis while eliminating the discomfort and risks associated with non-absorbable packing [4].
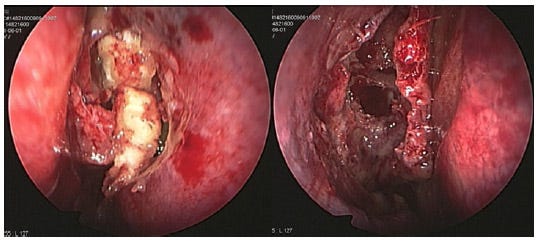
Fig. 4: Endoscopic findings of nasal packing immediately after functional endoscopic sinus surgery (FESS). In this patient, the left sinuses were packed with four pieces of dry Gelfoam pasted with terramycin ointment (left) and the right side was left without packing (right) [4].
Conclusion
As ENT surgery continues to advance toward minimally invasive and patient-friendly approaches, intranasal absorbable sponges for sinus surgery have become essential tools. With its strong haemostatic effect, high biocompatibility, and easy handling, SURGISPON® stands out as a reliable and economical option.
Whether used to control bleeding in FESS or to support grafts in tympanoplasty, SURGISPON® ensures optimal surgical outcomes and improved patient experience — making it a preferred alternative to traditional nasal packing.




Comments
Post a Comment